HELIOS-A Clinical Trial
HELIOS-A established the efficacy and safety of AMVUTTRA for hATTR‑PN
Global, randomized, open-label, multicenter, Phase 3 study1-2
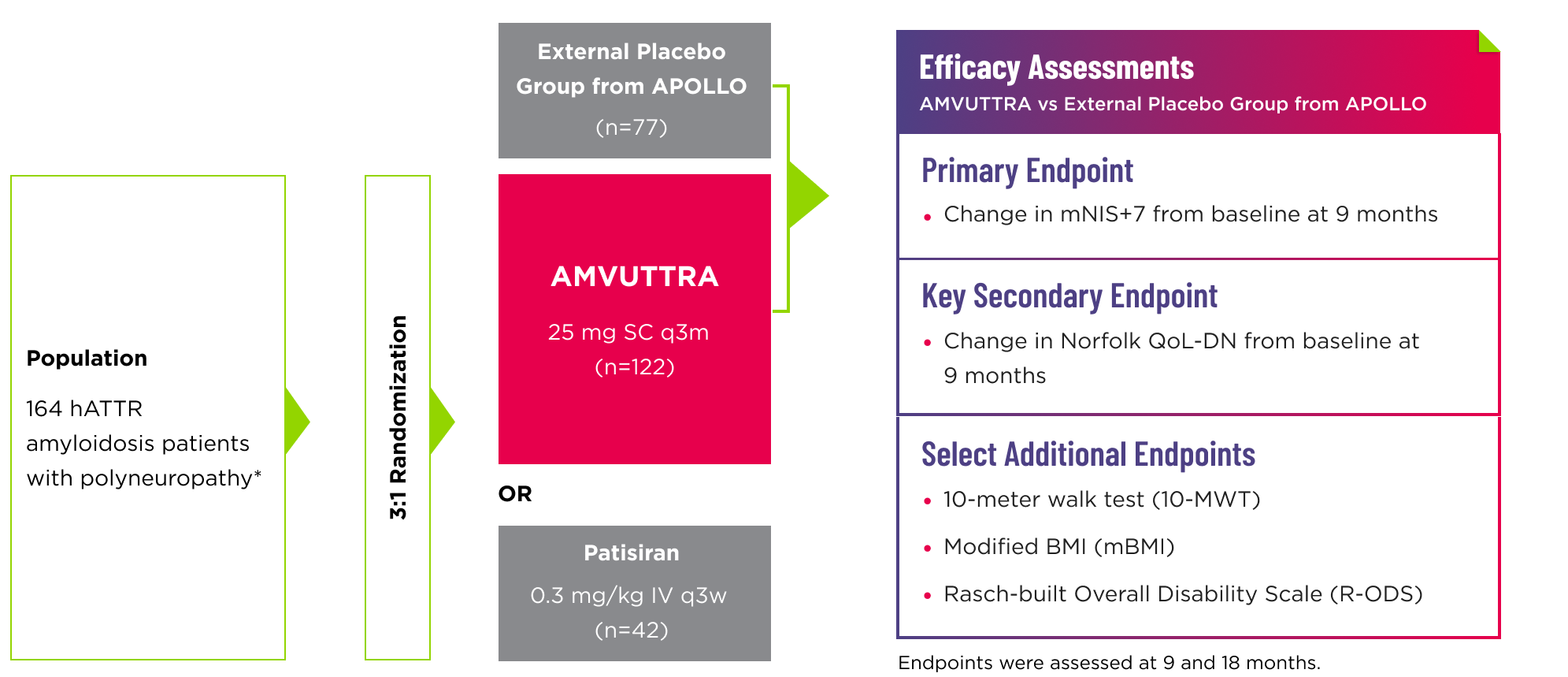
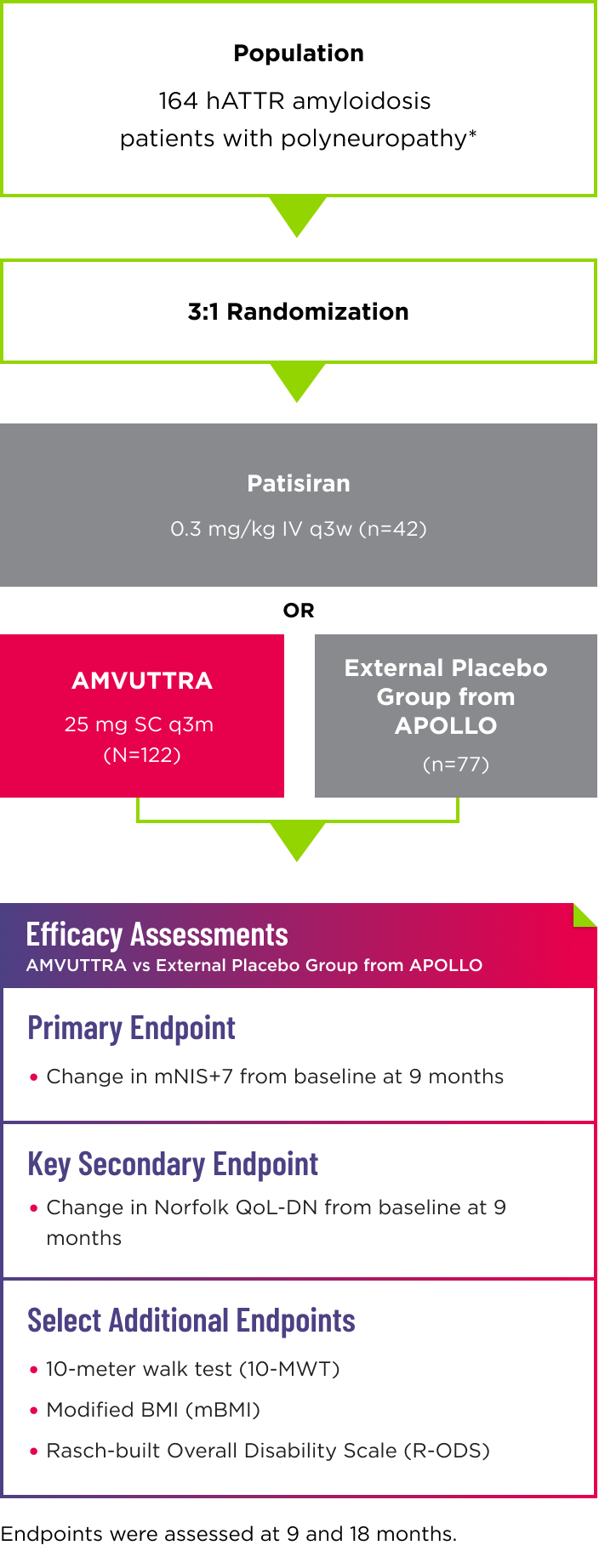
- Efficacy assessments were based on a comparison of the AMVUTTRA arm of the study with an external placebo group from APOLLO (n=77), a randomized controlled study in a comparable patient population with polyneuropathy caused by hATTR amyloidosis1,2*
- The patisiran reference group was included in the study to validate the use of the external placebo group3
- 96% of AMVUTTRA-treated patients completed at least 18 months of treatment2
*Study patients were ≥18 years of age, had a diagnosis of hATTR amyloidosis with polyneuropathy caused by any TTR variant, a Polyneuropathy Disability (PND) score ≤IIIb, a Neuropathy Impairment Score (NIS) of 5–130, a Karnofsky Performance Status (KPS) score ≥60%, and were permitted to have previously used TTR stabilizers.2
BMI=body mass index; hATTR=hereditary transthyretin-mediated amyloidosis; IV=intravenous; mNIS+7=modified Neuropathy Impairment Score + 7; Norfolk QoL-DN=Norfolk Quality of Life-Diabetic Neuropathy; q3m=every 3 months; q3w=every 3 weeks; SC=subcutaneous; TTR=transthyretin.
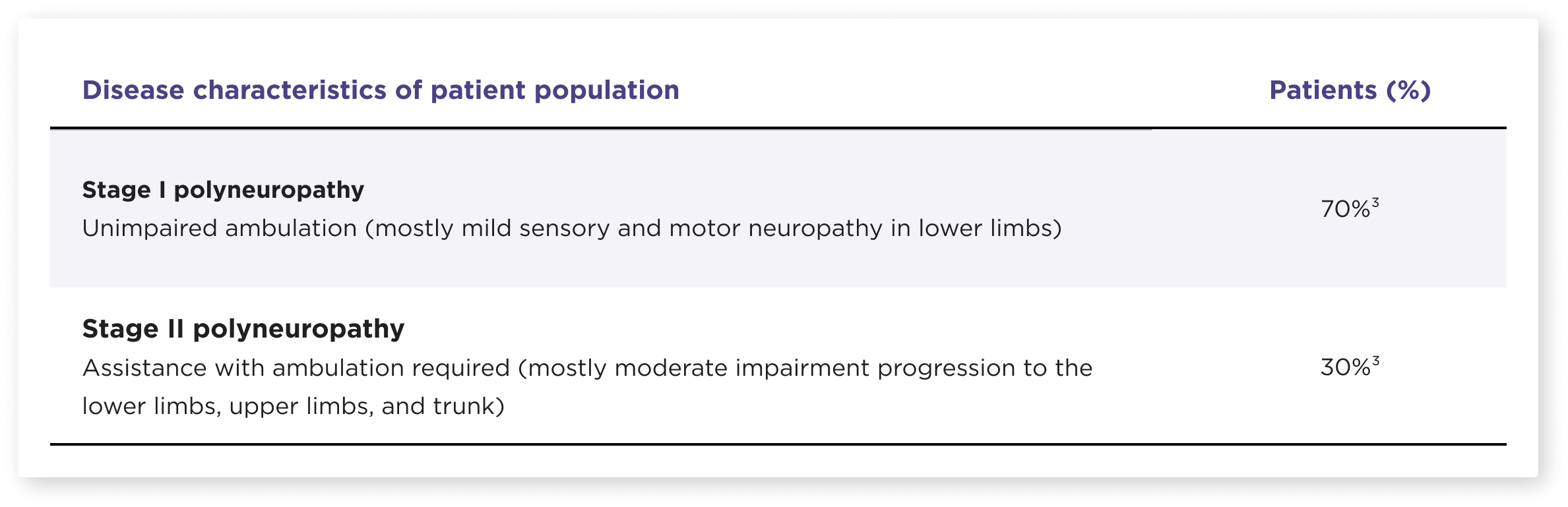
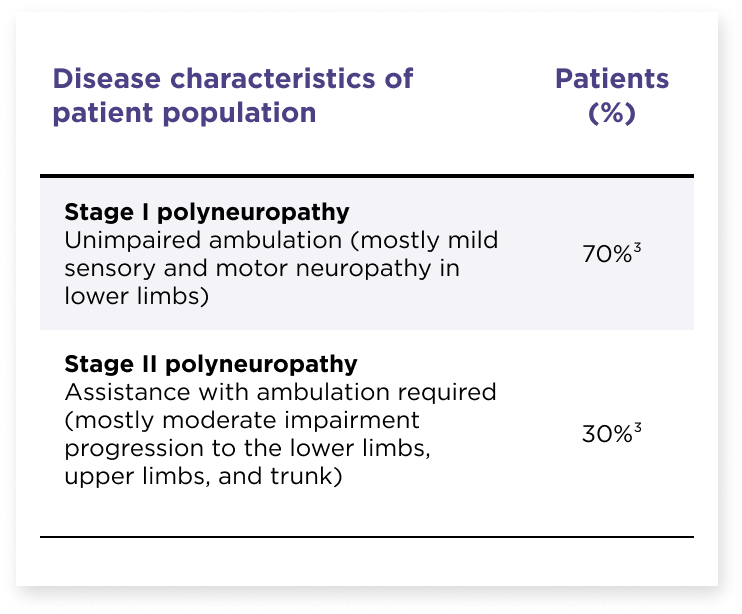
HELIOS-A included a diverse patient population1,2
Participants in the study were representative of the real-world population of patients with hATTR-PN2
Global study
164 patients from 22 countries2
Multiple variants
26 different TTR variants were representedV30M (45%), T60A (11%), E89Q (9%), A97S (5%), V122I (4%), S50R (3%), S77Y (3%), and D38A (3%)2,3
Broad age range
26 to 85 years old (median: 60 years old)2,3
Prior treatment
66% of patients had previously been treated with tafamidis or diflunisal2,3
Connect with a Rep
Speak with an Alnylam Representative to learn more about AMVUTTRA.
