HELIOS-B Clinical Trial
HELIOS-B was a landmark clinical trial establishing the efficacy and safety of AMVUTTRA® in ATTR-CM1
A global, randomized, double-blind, placebo-controlled, Phase 3 study1,2
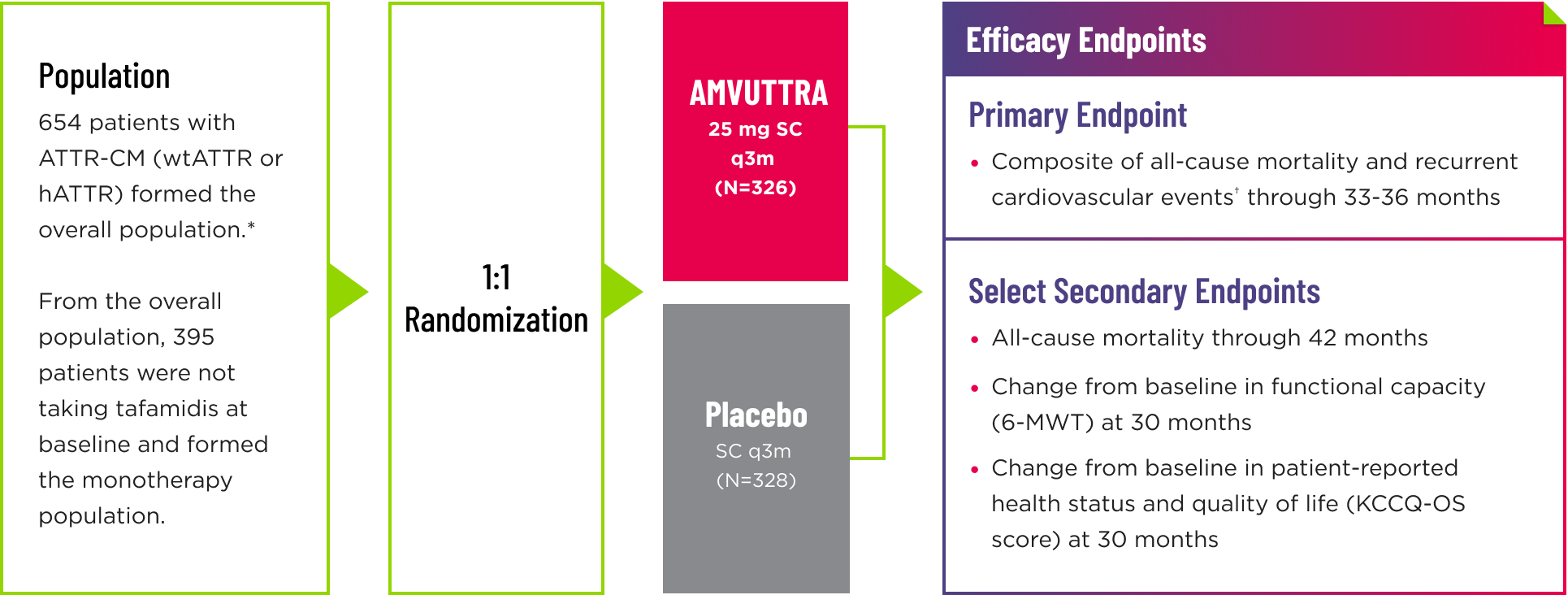
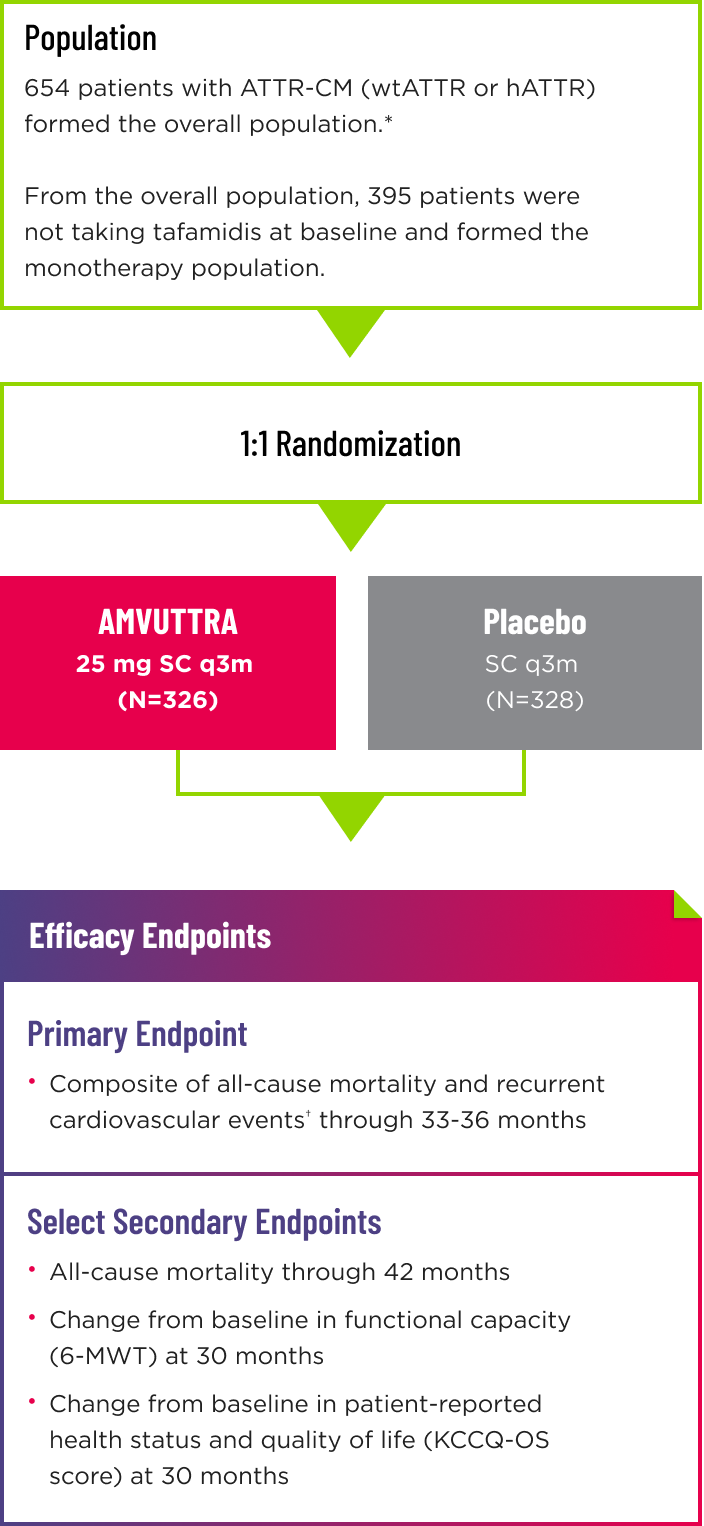
- Following the double-blind (DB) period of up to 36 months, patients on placebo were eligible to transition to AMVUTTRA in the open-label extension, which lasted up to 24 months2
- Randomization was stratified according to tafamidis use at baseline (with vs without), ATTR disease type (hereditary vs wild-type), and NYHA class and age at baseline (NYHA class I or II and age <75 years vs all others)1
*The overall population included patient cohorts with and without tafamidis use at baseline. 1
†Cardiovascular events are defined as hospitalizations for cardiovascular causes or urgent visits for heart failure. 1
6-MWT=6-minute walk test; ATTR=transthyretin-mediated amyloidosis; ATTR-CM=cardiomyopathy of transthyretin-mediated amyloidosis; hATTR=hereditary ATTR; mg=milligram; KCCQ‑OS=Kansas City Cardiomyopathy Questionnaire-Overall Summary; NYHA=New York Heart Association; q3m=every 3 months; SC=subcutaneous; wtATTR=wild-type ATTR.
One of the largest studies with a contemporary population of patients with ATTR-CM2,4,5*
The study population was typical of present-day patients with ATTR-CM2
Patient Characteristics at Baseline (Overall Population)3
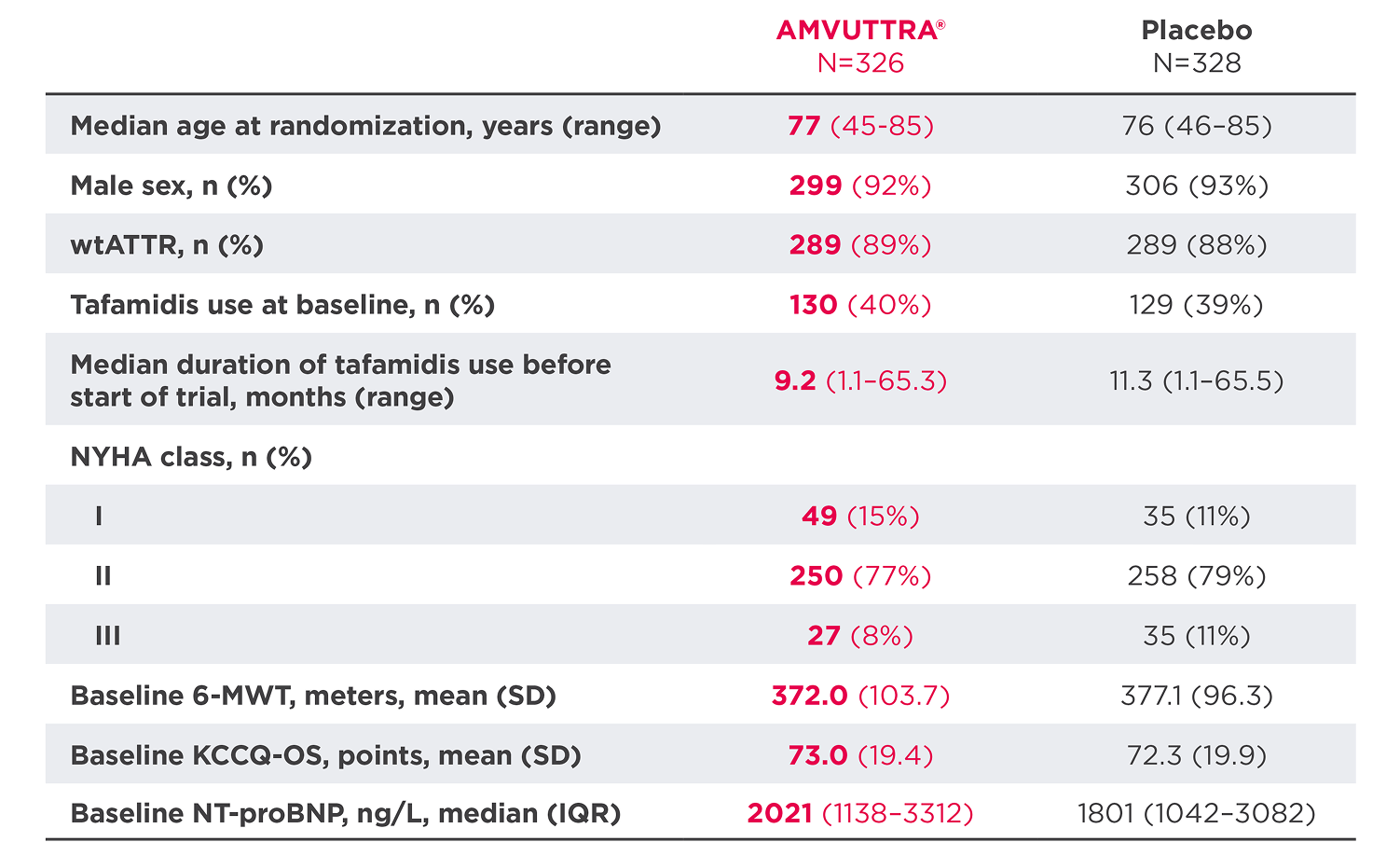
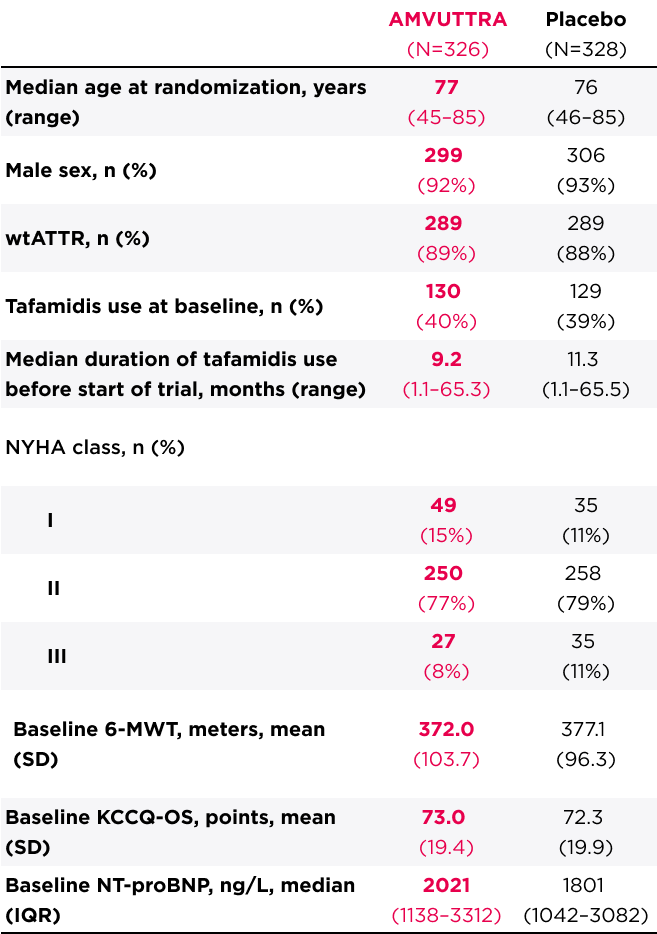
*Compared with other interventional pivotal studies for ATTR-CM.
IQR=interquartile range; NT-proBNP=N-terminal prohormone of brain-type natriuretic peptide; SD=standard deviation; SLGT2=sodium-glucose cotransporter-2.
HELIOS-B enrolled patients who were generally healthier than historical cohorts, as characterized by2,3:
Earlier diagnoses
Less severe disease
Increased heart-failure management
~40%were on tafamidis at baseline
~30%started SGLT2 inhibitors during the double-blind period
~80%were on diuretics at baseline
Connect with a Rep
Speak with an Alnylam Representative to learn more about AMVUTTRA.
