HELIOS-B Efficacy
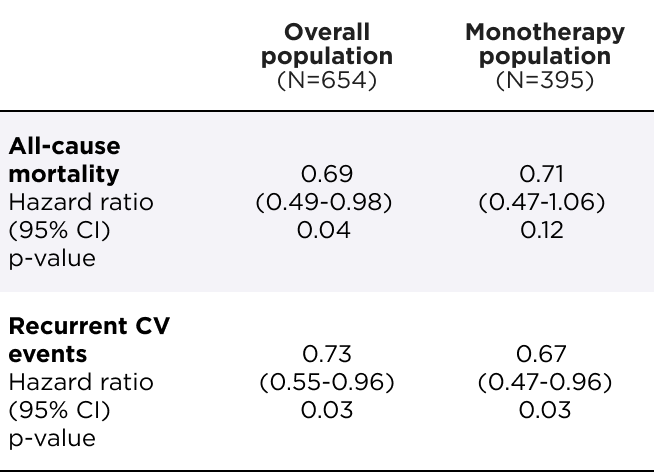
AMVUTTRA® significantly reduced the risk of ACM and recurrent CV events1
Primary composite endpoint analysis through 33-36 months1
Relative risk reduction
of ACM and recurrent CV events
in the overall population
(N=654)
[HR=0.72 (95% CI: 0.55–0.93); p=0.01]
Relative risk reduction
of ACM and recurrent CV events
in the monotherapy population
(N=395)
[HR=0.67 (95% CI: 0.49–0.93); p=0.02]




*The Kaplan-Meier curves are unadjusted for baseline imbalances in disease-severity characteristics.3
†Heart transplantation and left ventricular assist device placement are treated as death. HR and 95% CI are based on a Cox proportional-hazards model.1
‡Data were censored at each patient's first dose in the OLE, which could occur at approximately 33 or 36 months, depending on the patient's enrollment time. For patients who did not enter the OLE, data were censored at their study discontinuation date.3
CI=confidence interval; CV=cardiovascular; DB=double-blind; HR=hazard ratio; OLE=open-label extension.
AMVUTTRA achieved consistent results across all prespecified subgroups1


At the end of the DB period, all remaining patients on placebo transitioned to AMVUTTRA treatment in the OLE.3


*The Kaplan-Meier curves are unadjusted for baseline imbalances in disease-severity characteristics.3
†Heart transplantation and left ventricular assist device placement are treated as death.5
‡For patients in both treatment arms, survival time was censored 6 months after the first dose in the OLE period.3
¶Analysis conducted in 2024.
CI=confidence interval; HR=hazard ratio.
Significant benefit observed in functional capacity and quality of life for patients treated with AMVUTTRA compared with placebo1,5


- Healthy adults without ATTR-CM experience a natural decline in 6‑MWT of 5‑6 meters per year6
- A 5-point change in KCCQ‑OS (a measure of health status and health‑related QoL) is considered clinically meaningful7
*6-MWT assesses functional capacity by measuring distance walked over a period of 6 minutes. A decrease in the distance walked indicates a decline in functional capacity.8
†The KCCQ is a 23-item, self-administered questionnaire that measures the patient’s perception of health status within a 2-week recall period (scoring on KCCQ-OS: 0 to 100, with a higher score indicating better quality of life).3
6-MWT=6-minute walk test; CI=confidence interval; KCCQ-OS=Kansas City Cardiomyopathy Questionnaire-Overall Summary; LS=least squares; SE=standard error.
Patients receiving AMVUTTRA maintained relative stability in functional capacity and quality of life3




QoL=quality of life.
Connect with a Rep
Speak with an Alnylam Representative to learn more about AMVUTTRA.

